
Six aqueous solutions containing increasing concentrations of potassium permanganate
BIOL 1406
PreLab 2.1
What is the difference between the amount and the concentration of solutes in a solution?
|
|
A solution is a homogeneous mixture composed of two or more substances. The substances can be solids, liquids, or gasses. The substance that makes up most of the solution is called the solvent. If the solvent is water, then the solution is called an aqueous solution. The minor components of the solution are called solutes. For example, if we dissolve 2 grams of sugar and 4 grams of salt in 2 L of water, water is the solvent while sugar and salt are solutes. This would be an example of an aqueous solution. Since living organisms are composed mostly of water, studying the chemistry of life largely involves studying the chemistry of aqueous solutions. Therefore, preparing aqueous solutions is an essential skill in molecular and cellular biology labs. |
|
Six aqueous solutions containing increasing concentrations of potassium permanganate |
The amount of solute in a solution refers to the total quantity of solute in the solution. It is generally measured by weight (using units such as grams) or by volume (using units such as milliliters). Concentration, on the other hand, refers to the amount of solute per unit of solution. Concentration can be expressed as a ratio:
|
Concentration of solution = amount of solute / amount of solution |
For example, if we have 10g of sucrose in 2L of solution, the amount of solute in this solution is 10 g. The concentration of solute in this solution, on the other hand, is 10g/2L, or 5 g/L, read as “5 grams per liter.” If we multiply the amount of solution by the concentration of the solution, this will give us the total amount of solute present. In our example:
|
(2L) x (5g/L) = 10g of solute |
Another way to describe the concentration of a solution is by percent weight per unit volume. This equals the number of grams of solute per 100 mL of solution. For example, if we have 10g of sucrose in 2L of solution, this equals 5g in 1 L of solution, or 5g in 1,000 mL, or 0.5g in 100 mL, or a 0.5% sucrose solution.
|
A third way to describe the concentration of a solution is by molarity. With molarity, the amount of solute present is measured in units called moles rather than grams or milliliters. How much is a mole? One mole (1 mol) of any substance is equal to 6.02 x 1023 basic units. (Note: the basic units of an element are individual atoms, and the basic units of a compound are individual molecules.) The molarity of a solution tells us the number of moles of solute per liter of solution. Note that:
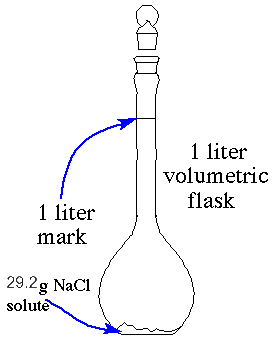
How can we measure out one mole of a solute? The formula weight of a substance tells us how many grams one mole of that substance weighs. For example, the formula weight of potassium permanganate (KMnO4) is 158.04 g/mole. Therefore, if you have 158.04 g of potassium permanganate in one liter of solution, the concentration would be 1 mole per liter or 1 molar (1M). The figure to the right shows how to make 1 liter of a 0.5 M solution of NaCl (formula weight 58.4 g/mole). {58.4 g/mol x 0.5 mol/L = 29.2 g/L of NaCl; when 29.2 g of NaCl is diluted with water to the one liter mark you will have 0.5 moles of NaCl in 1 liter of solution or a 0.5 M solution.} |
 |
| IMPORTANT: Please note that “gram”, “mg”, “liters”, “mole”, and “millimole” are all units of amount. On the other hand, “g/L”, “mg/mL”, “millimoles per liter”, “micromolar”, and “molar” are all units of concentration. Whenever you report the amount of a substance or the concentration of a solution, make sure you use appropriate units. Also note that the abbreviation for mole (a unit of amount) is “mol” and the abbreviation for molar (a unit of concentration) is “M”. |
When making solutions for use in the biology lab, controlling the concentration of solutes in the solution is often critical. For example, failure to carefully control the concentration of solutes in solutions used to culture cells can cause the cells die or develop abnormally. Likewise, inappropriate concentrations of solutes in solutions that contain biomolecules may cause the biomolecules to denature or aggregate, thus making them inactive. Concentrations of solutes can also affect the speed and types of chemical reactions that take place within a solution.
| YOUR TURN | ||
Enter your answers in the spaces provided. You can move your cursor over the hotspots to get a "Hint" or "Check your answer." Don't forget to utilize what you learned in the previous lab about significant digits as you work these problems. |
||
| What is the molarity of a solution that contains 34 grams of potassium permanganate in 758 ml of solution? | Hint | Check your answer. |
| What is the percent weight per unit volume of a sucrose solution that contains 59 grams of sucrose in 1700 ml of solution? | Hint | Check your answer. |
| How many grams of potassium permanganate are needed to prepare 1 L of a 2 mM solution? | Hint | Check your answer. |
Close this browser window to return to Blackboard and complete the practice quiz and assessment quiz.