BIOL 1406
PreLab 2.2
How can I use a spectrophotometer to determine the concentration of solutes in a solution?
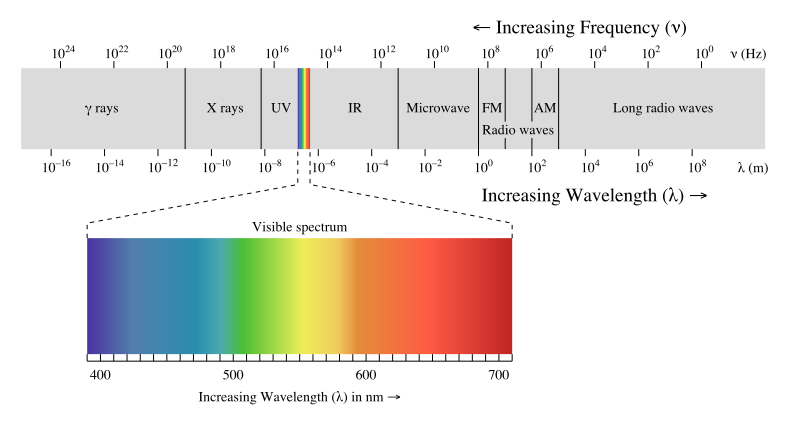
Electromagnetic radiation is a form of energy that travels through space with the characteristics of a wave. Electromagnetic radiation with wavelengths between about 400 nm and 740 nm is detected as visible light. Our eyes detect different wavelengths of visible light as different colors. For example, light with a wavelength of 400 nm appears violet while light with a wavelength of 740 nm appears red. White light is actually a mixture of all the visible wavelengths. When white light passes through a solution, the color of the solution will depend on how much of each wavelength passes through the solution (called optical transmittance) and how much of each wavelength is absorbed (called optical absorbance).

|
A spectrophotometer is an instrument that can pass
light of a single wavelength through a solution and measure the amount that
passes through. First, the spectrophotometer must be zeroed by passing light of
the chosen wavelength (500 nm for example) through a blank containing only the
solvent. Then, the same wavelength of light is passed through the solution.
The percentage of light that passed through the solution relative to the amount
that passed through the solvent alone is called the percent transmittance at
500 nm. For example, if half as much light passes through a solution as
passes through the solvent alone, we would record this as 50% T: |
|
Percent Transmittance (%T) = (amount of light transmitted through the solution / amount of light transmitted through the solvent) x 100 |
On the other hand, the amount of light at 500 nm that is
absorbed by the solution is called the absorbance at 500 nm and is
abbreviated A500. Absorbance is the negative logarithm of the
percent transmittance divided by 100. Because logarithms have no units,
absorbance has no units:
|
Absorbance (A) = - log (% transmittance / 100) |
|
|
|
It is extremely important that you
know how to calibrate the Spec-20 and use it to measure absorbance. |
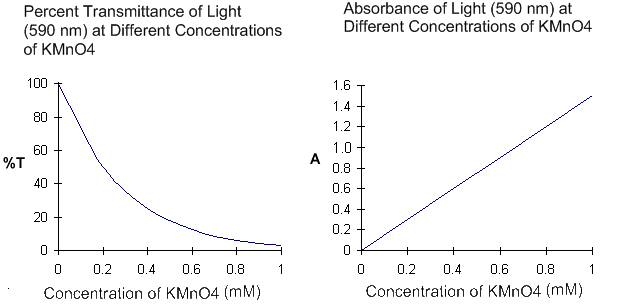
| Examine the graphs of percent transmittance and absorbance shown on the right. Notice that these 2 variables are inversely related to each other; in other words, they move in opposite directions. For example, if solute concentration is increasing, percent transmittance will decrease while absorbance will increase. Also note that the absorbance of a solution is linearly related to its concentration. This means that if the concentration of a solution increases at a steady rate, then absorbance will also increase at a steady rate (within limits.) This is called a linear relationship because if we measure the absorbance of several solutions with different solute concentrations, and then plot a graph with solute concentration on the x-axis and absorbance on the y-axis, our data points should fall on a straight line. |
 |
In this lab, you will learn 3 methods for preparing
solutions that contain a specific concentration of solutes. You will then use
all 3 methods to prepare 14 solutions of KMnO4, each with a different
concentration of solute. Next, you will measure the absorbance of each solution
with a spectrophotometer and plot a graph of your data with the "solution
concentration" on the x-axis and the "absorbance" on the y-axis. If your
solutions were prepared correctly, all of your data points should fall on a
straight line. Then you will use a statistical technique called linear
regression to determine the equation of the "best-fit" straight line.
This equation will tell you the exact mathematical relationship between the 2
variables (solute concentration and absorbance). Finally, you
will use this equation to estimate the concentration of a solution of potassium
permanganate of unknown concentration.
| YOUR TURN | ||
| The following scatter diagram was prepared by plotting "Concentration
of KMnO4 (mM)" vs. "A 540 values" for 7 solutions. The data
points are shown as dots. A linear regression line was then calculated
from the data points and plotted as a black line:
Examine the scatter diagram carefully and then answer the questions below: |
||
| Different colors of visible light have different . | Hint | Check your answer. |
| What is a spectrophotometer used for? | Hint | Check your answer. |
| What is the difference between percent
transmittance (%T) and absorbance (A)? |
Hint | Check your answer. |
| If a solution has a percent transmittance of 20% at 595nm, what is its optical absorbance at 595 nm? | Hint | Check your answer. |
| A solution has a percent transmittance of 30% at 595nm. As you make the solution more and more dilute, what happens to its percent transmittance at 595nm? | Hint | Check your answer. |
| A solution has an absorbance of 0.62 at 595nm. As you make the solution more and more dilute, what happens to its absorbance at 595nm? | Hint | Check your answer. |
| According to the linear regression line, what is the predicted A540 value for a 0.6 mM KMnO4 solution? | Hint | Check your answer. |
| According to the linear regression line, what is the predicted concentration for a KMnO4 solution with an A540 value of 0.4? | Hint | Check your answer. |
Close this browser window to return to Blackboard and complete the practice quiz and assessment quiz.