BIOL 1406
PreLab 2.4
How can I prepare solutions with specific solute concentrations using the parallel dilution technique?
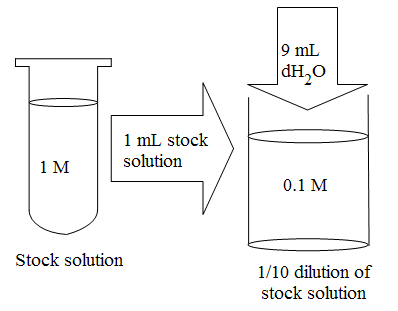
When preparing solutions, it is often easier to dilute an existing stock solution than it is to weigh out a solid solute and then dissolve it in the solvent. This is especially true if you want to prepare several solutions that each have a different concentration of the same solute.

One way to dilute an existing stock solution is by using the parallel dilution technique. When using this technique, first calculate the amount of stock solution needed to make your dilution.
|
When making parallel dilutions, use the following formula to calculate the amount of stock solution needed for each dilution: |
|
C1 X V1 = C2 X V2 or C1V1 = C2V2 |
|
For example, if you want to make 50 mL of 0.15 M calcium chloride (CaCl2) from a 2.0 M stock solution:
C1 = 2.0 M
C2 = 0.15 M and
V2 = 50 mL
To determine the volume of stock solution required (V1):
C1V1 = C2V2
(2.0 M) V1 = (0.15 M ) ( 50 mL )
(2.0 M) V1 = 7.5 M mL
V1 = 3.75 mL
After you have calculated the required amount of stock solution (V1), pour the stock solution into a graduated cylinder and then add enough dH2O to bring the final volume up to 50 mL. Alternatively, you can pour the required amount of stock solution into a container, and then calculate and add the amount of dH2O needed to give you a final volume of 50 mL. (In this example, 50 mL minus 3.75 mL of stock solution equals 46.25 mL, so 46.25 mL of dH2O must be added to the 3.73 mL of stock solution.)
| YOUR TURN | ||
|
Calculate the amount of
0.01 M potassium permanganate (KMnO4)
stock solution needed to prepare 25 mL of a 2 mM solution. (Note that 2 mM
= 0.002 M.) Write your answer in the space below and in your lab
notebook. Volume of stock solution needed Don't forget the units! |
Hint | Check your answer. |
Sometimes you must prepare several solutions that each have a different concentration of the same solute. This is referred to as a dilution series. When preparing a parallel dilution series, use the formula C1V1 = C2V2 to calculate how much stock solution is needed to make each dilution.
| YOUR TURN | |||||
|
Calculate the amount of 0.01 M potassium permanganate (KMnO4) stock solution and the amount of dH2O needed to make 10 mL each of the following dilutions. Write your answers in the spaces below and in your lab notebook. |
|||||
| Diluted Concentration | Volume of 0.01 M KMnO4 Stock Solution Needed | Volume of dH2O needed | |||
| 1.0 mM KMnO4 | Hint | Check your answer. | Check your answer. | ||
| 0.6 mM KMnO4 | Hint | Check your answer. | Check your answer. | ||
| 0.4 mM KMnO4 | Hint | Check your answer. | Check your answer. | ||
| 0.2 mM KMnO4 | Hint | Check your answer. | Check your answer. | ||
| 100 μM KMnO4 | Hint | Check your answer. | Check your answer. | ||
| 50 μM KMnO4 | Hint | Check your answer. | Check your answer. | ||
| 20 μM KMnO4 | Hint | Check your answer. | Check your answer. | ||
Close this browser window to return to Blackboard and complete the practice quiz and assessment quiz.