BIOL 1406
PreLab 2.5
When do I use the serial dilution technique instead of the parallel dilution technique?
Another way to make dilutions is to use some of your existing stock solution to make a dilute solution, then use some of the dilute solution to make an even more dilute solution, then use some of that solution to make an even more dilute solution, and so on. This procedure is called the serial dilution technique.

There are two situations where serial dilutions should be used rather than parallel dilutions:
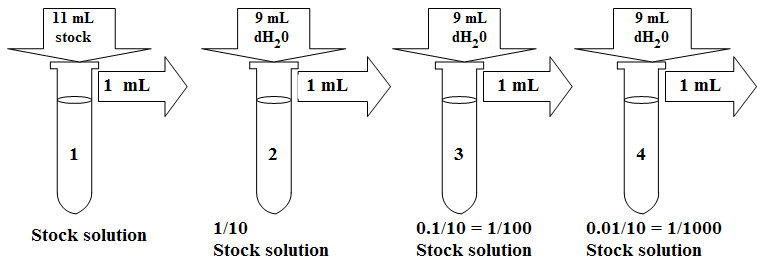
FIRST: Use a serial dilution when you need several solutions of the same solute and there is a constant dilution factor. For example, suppose you have a 2 M stock solution of KMnO4 and you want to make 15 mL of each of the following concentrations of KMnO4: 0.2 M, 20 mM, 2 mM, and 0.2 mM. Notice that the concentration of each solution is 1/10th the concentration of the previous solution in the series. The factor by which each solution is diluted compared to the previous one is called the dilution factor.
SECOND: Also use a serial dilution when the dilution factor is so large that the amount of stock solution needed to make the dilution in one step (using the formula C1V1 = C2V2) is too small to measure accurately. Remember that the smallest volume you can measure with the micropipettors is 2 μL.To calculate the dilution factor for each dilution, divide the concentration of the starting solution by the concentration of the diluted solution. For example, for the first dilution 2 M divided by 0.2 M equals 10. For the second dilution, 0.2 M divided by 20 mM equals 10. For the third dilution, 20 mM divided by
2 mM equals 10. And for the fourth dilution 2 mM divided by 0.2 mM equals 10. Therefore, this series has a constant dilution factor of 10.
| YOUR TURN | ||
| You have a stock solution of 1.6 M sucrose and you need to prepare solutions with the following concentrations of sucrose: 0.4 M, 0.1 M, 25mM, and 6.25 mM. What is the dilution factor for this series? | Hint | Check your answer. |
|
You plan to prepare 4 solutions by serial dilution. The concentration of your stock solution is 2.7 M and the dilution factor is 3. What will be the molarity of your four solutions? |
Hint | Check your answer. |
| You have a 1.0 M stock solution of glycine (an amino acid), and you need 5 mL of a 0.1 mM solution. By what factor do you need to dilute your stock solution? | Hint | Check your answer. |
| Using the formula C1V1 = C2V2, calculate the amount of stock solution you need to make 5 mL of a 0.1 mM solution from a 1.0 M stock solution. Express your answer in mL: | Hint | Check your answer. |
| Do you have a measuring device that will accurately measure this amount? YES NO | Hint | Check your answer. |
|
Explain.
|
Hint | Check your answer. |
| YOUR TURN | ||
|
For each of the following situations, state whether the parallel or serial dilution technique would be more appropriate, and explain your reasoning. |
||
You need 20 mL of a 5 mM glycerol solution. You have a 1.0 M glycerol stock solution on hand. Which dilution technique is appropriate and why?
|
Hint | Check your answer. |
You have a 2.5 M stock solution of EDTA. You need 50 mL each of 2 M, 0.5 M, 0.2 M and 0.1 M solutions of EDTA. Which dilution technique is appropriate and why?
|
Hint | Check your answer. |
You have a 2.0 M stock solution of sucrose. You need 100 mL each of 1 M, 0.5 M, 0.25 M, 0.125 M, and 62.5 mM sucrose solutions. Which dilution technique is appropriate and why?
|
Hint | Check your answer. |
Close this browser window to return to Blackboard and complete the practice quiz and assessment quiz.