![]() NOTE: The letters below correspond to the letters on the
arrows in the diagram above.
NOTE: The letters below correspond to the letters on the
arrows in the diagram above.
BIOL 1406
PreLab 2.6
How can I prepare solutions with specific solute concentrations using the serial dilution technique?
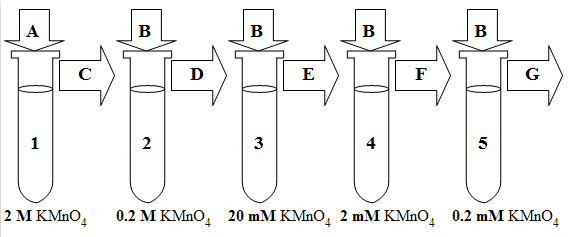
Letís return to our example where you have a 2 M stock solution of KMnO4 and you want to make 15 mL of each of the following concentrations of KMnO4: 0.2 M, 20 mM, 2 mM, and 0.2 mM. In this case you must make a total of 4 dilutions.
First, the 2 M stock solution is diluted to make the 0.2 M solution.
Next, the 0.2 M solution is diluted to make the 20 mM solution.
Next, the 20 mM solution is diluted to make the 2 mM solution.
Finally, the 2 mM solution is diluted to make the 0.2 mM solution.
How would you make these 4 dilutions using the serial dilution technique? Before you begin, you must determine the values of 3 variables: df, v2, and v1.
| df | For each dilution, df is the dilution factor. We have already seen that this dilution series has a constant dilution factor of 10. |
| v2 | For each dilution, v2 is the volume of diluted solution that you want to make. Since we want to make 15 mL of each diluted solution, v2 = 15 mL |
| v1 | For each dilution, v1 is the volume of more concentrated solution that you mix with solvent to make the more dilute solution. If you know df and v2, you can calculate the value of v1 using the following formula: |
|
When making serial dilutions, use the following formula to calculate the value of v1: (v1 + v2) / v1 = df |
In our example:
(v1 + 15 mL) / v1 = 10
v1 + 15 mL = 10 v1
15 mL = 10 v1 - v1
15 mL = 9 v1
v1 = 15 mL / 9
v1 = 1.67 mL
Once you know the values of df, v2, and v1, set up 5 tubes (one for the stock solution and one for each of the dilute solutions) as shown in the following diagram and then follow the procedure described below the diagram:
|
|
| YOUR TURN | ||
|
Starting with a stock solution of 2 mM KMnO4, you plan to make 6 mL of each of the following concentrations of KMnO4: 1.0 mM, 0.5 mM, 250 μM, 125 μM, 62.5 μM , 31.3 μM , and 15.6 μM |
||
| What is df for this dilution series? | Hint | Check your answer. |
| What is V2 for this dilution series? | Hint | Check your answer. |
| What is V1 for this dilution series? | Hint | Check your answer. |
| Examine the figure below and answer the questions in the
spaces provided. You will
need this information during lab, so you should also record it in your lab
notebook.
|
||
| What is the volume of "A" placed into tube 1? | Hint | Check your answer. |
| What is the volume of solvent "B" placed into tubes 2-8? | Hint | Check your answer. |
| What is the volume of "C to I" transferred between tubes 1 - 8? | Hint | Check your answer. |
| What is the final volume of tubes 1-8? | Hint | Check your answer. |
| What is the volume of "J" that is discarded? | Hint | Check your answer. |
|
You have now learned three methods for preparing solutions. Use the interactive exercise below to test yourself and see if you can choose the best method and correctly carry out the procedure to make the required aqueous solutions. |
Close this browser window to return to Blackboard and complete the practice quiz and assessment quiz.