The pH scale was developed to measure how acidic or basic (alkaline) a
solution is. The pH of a solution is defined as the negative logarithm, to the
base 10, of the hydrogen ion concentration of the solution:
NOTE: Like absorbance, pH is the logarithm of a number and therefore has no units. A logarithm (or log) is the power to which a base, in this case 10, must be raised to give the desired number. For example, the log of 100 is 2 (to get 100, we must raise 10 to the power of 2) and the log of 1,000 is 3 (to get 1,000, we must raise 10 to the power of 3). A neutral solution has a hydrogen ion concentration of 1 x 10-7 M and a hydroxyl ion concentration of 1 x 10-7 M. The logarithm of 1 x 10-7 is -7, so the negative logarithm of 1 x10-7 is 7. Therefore, the pH of a neutral solution is 7.
|
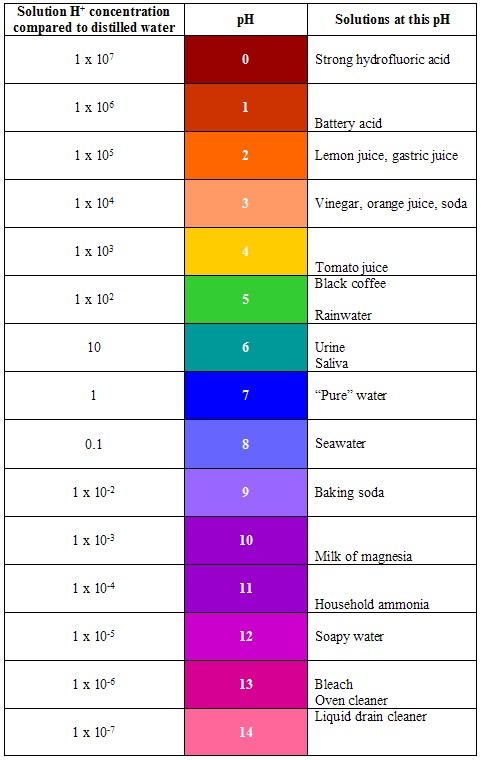
The pH Scale |
|
|
|
||
|
Scientists use a variety of methods to measure pH. Some methods rely on chemical indicators that turn a specific color depending on the pH of the solution. A more precise method involves use of an electronic pH meter. Like all electronic measuring devices, a pH meter must be calibrated before any readings are taken, in order to ensure accurate results. Click the play arrow above to watch a short video about how to calibrate the pH meter and use it to measure the pH of a solution. If you have problems seeing the video, you can view it in an External Viewer |