BIOL 1406
PreLab
7.4
How can a scanning spectrophotometer be used to analyze the
purified pigments that were separated using TLC?
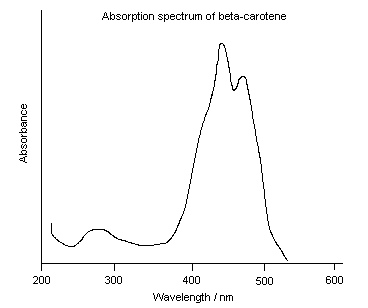 |
In Exercise 2, you learned that a spectrophotometer can be used to shine a
specific wavelength (color) of light through a solution and then measure how
much of the light is absorbed. This measurement is referred to as the absorbance
at that wavelength. In today’s lab you will use a special type of
spectrophotometer, called a scanning spectrophotometer, to automatically measure
the absorbance of a solution at many different wavelengths. The results are then
plotted to form a curve called an absorption spectrum, like the one for
beta-carotene shown to the left:
An absorption spectrum shows which wavelengths of light are most strongly
absorbed by a solution (peaks on the curve) and which wavelengths are most
weakly absorbed (low points on the curve). Wavelengths where peaks occur are
called wavelength maxima or absorption maxima. |
Because each substance has a characteristic absorption spectrum, examining the
absorption spectrum of a solution can help determine what substances are present
in the solution and whether any contaminants are present. When examining an
absorption spectrum for purposes of identification, it is important to focus on
the location of the absorption peaks and valleys (i.e. at which wavelengths the
peaks and valleys occur) and NOT on the heights of the peaks. This is because a
given substance will always have peaks at the same locations (wavelengths), but
the heights of the peaks may vary depending on how concentrated the solution is.
Below you can see the absorption spectra for 2 different pigments, chlorophyll a
and chlorophyll b, plotted on the same axis. Note that each type of chlorophyll
has its own characteristic absorption spectrum:
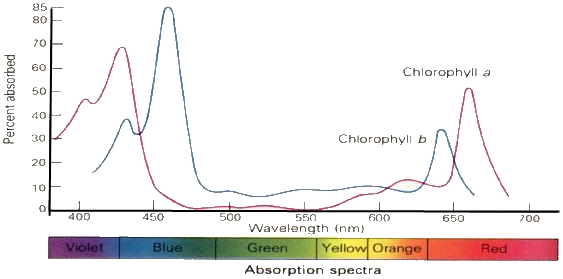
Close this browser window to return
to Blackboard and complete the practice quiz and assessment quiz.

